Abortion rights, women of color, and LGBTQIA+ people are under attack. Pledge to join us in fighting for gender justice.
HOT DAMN! The FDA Approves the First-Ever Over-the-Counter Birth Control Pill in the U.S.
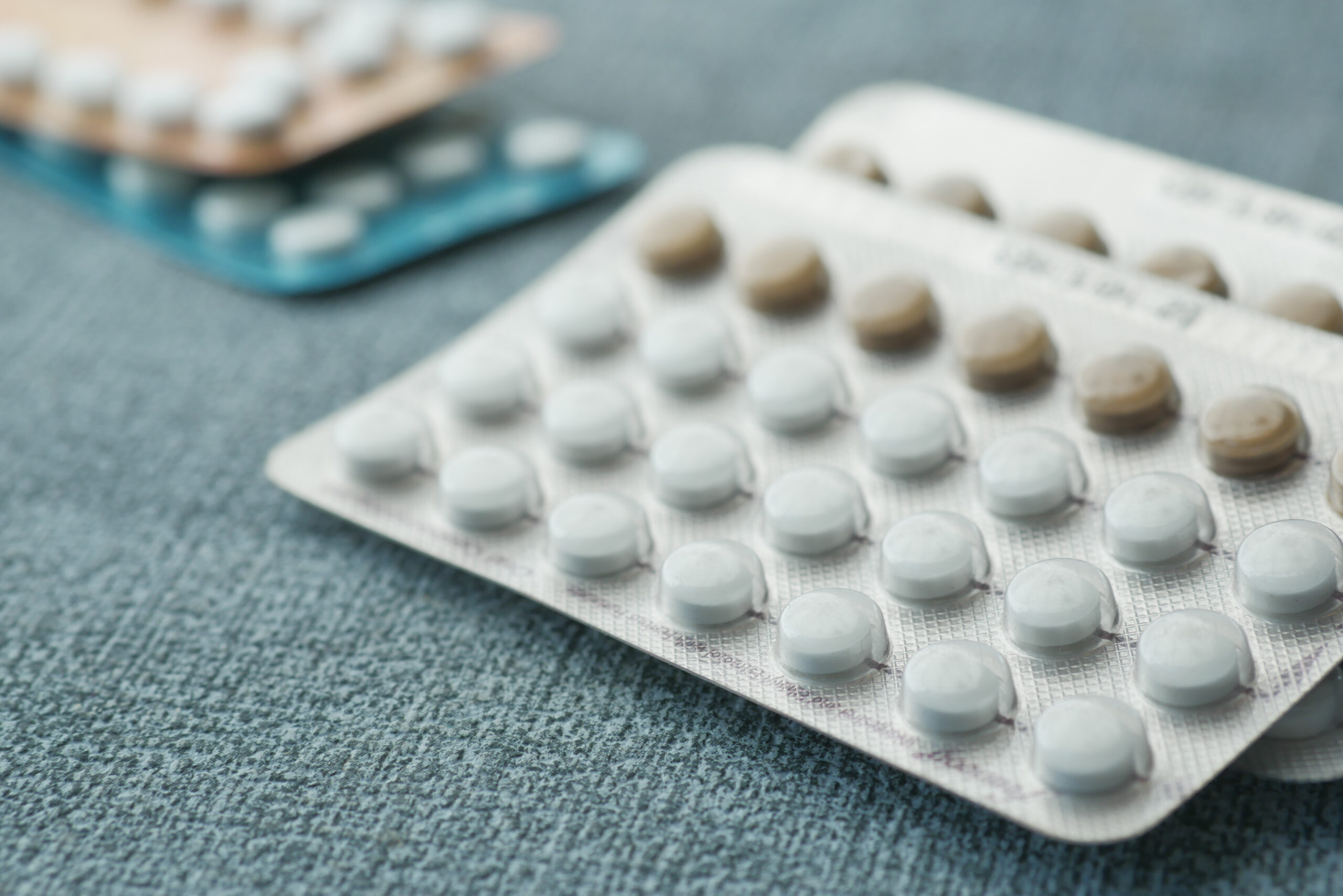
History has been made! Last week, the Food and Drug Administration (FDA) approved Opill as the first daily oral contraceptive in the U.S. that can be accessed without a prescription. This is a monumental moment for reproductive health. An over-the-counter (OTC) pill will undoubtedly reduce barriers that prevent people from accessing contraception.
If you’re hearing the name “OPill” for the first time, let me give you a brief refresher.
Opill (norgestrel) is a progestin-only pill that was originally approved by the FDA in 1973 for prescription use. For years, Free the Pill, a coalition of more than 200 reproductive health, rights, and justice advocates, has worked tirelessly to get a daily oral contraceptive approved for nonprescription use in the United States – conducting research, raising awareness of why over-the-counter access is crucial, and setting the stage for this amazing day. In 2022, HRA Pharma (the manufacturer of Opill) submitted its first-ever application to the FDA to switch Opill from prescription to over-the-counter status. In May of 2023, the FDA held an Advisory Committee hearing to discuss the decades of science and research showing that birth control pills are safe and effective for over-the-counter use for individuals of all ages. At the hearing, the FDA Advisory Committee voted unanimously to recommend Opill to be offered OTC, and the FDA’s final decision last week confirmed that.
With the great news of Opill’s approval, we will likely see the pill stocked on shelves at our local stores in January 2024. In the meantime, Free the Pill and its coalition of members will continue to work to make sure that it can be accessed once it hits the shelves, and that people will have the information they need to determine if Opill is the right choice for them.